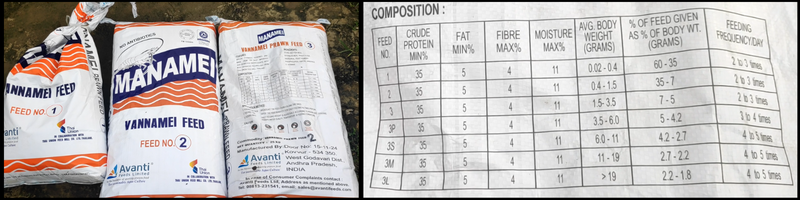
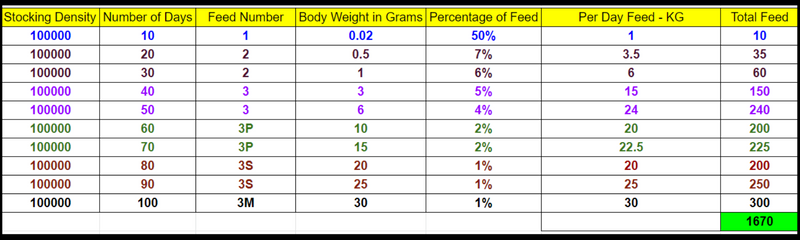
pH Vs Alkalinity – A Detail Explanation
pH and alkalinity are both important measures of water quality, but they are distinct parameters that measure different aspects of water chemistry.
pH is a measure of the acidity or basicity of a solution, and it is defined as the negative logarithm of the hydrogen ion concentration ([H+]) in a solution. The pH scale ranges from 0 to 14, with 7 being neutral. A pH value below 7 is acidic, and a pH value above 7 is basic. The pH of natural water sources can vary widely depending on factors such as the geology of the surrounding area, the presence of dissolved gases, and the activity of aquatic organisms.
Alkalinity is a measure of the water’s ability to
resist changes in pH, and it is determined by the concentration of bicarbonate (HCO3-), carbonate (CO32-), and hydroxide (OH-) ions in the water. Alkalinity is expressed as the equivalent amount of calcium carbonate (CaCO3) in milligrams per liter (mg/L). Alkalinity is an important parameter for maintaining stable pH levels in aquatic environments, as it acts as a buffer to prevent large changes in pH due to the addition of acids or bases.
While pH and alkalinity are related, they are not the same thing. A solution can have high alkalinity but a low pH, or vice versa. For example, a solution with a high concentration of bicarbonate ions can have high alkalinity, but if it is exposed to an acid, the pH can still drop significantly. Similarly, a solution with a low alkalinity but a high concentration of hydroxide ions (a strong base) can have a high pH.
In natural aquatic systems, pH and alkalinity are often closely linked. In general, surface waters tend to have a pH range of 6.5 to 8.5, with alkalinity levels that vary depending on the local geology and the amount of dissolved minerals in the water. In some areas, particularly in regions with limestone or other carbonate rock formations, the alkalinity of the water can be very high. This is because the carbonates in the rock dissolve in the water and increase the concentration of bicarbonate and carbonate ions, which in turn increases the alkalinity.
In summary, pH and alkalinity are both important parameters for understanding water quality. pH measures the acidity or basicity of a solution, while alkalinity measures the water’s ability to resist changes in pH. Both pH and alkalinity can be affected by a wide range of factors, including geology, climate, and human activities such as pollution and land use changes. By monitoring pH and alkalinity levels in natural water systems, researchers and resource managers can better understand the health of these ecosystems and take steps to protect them.