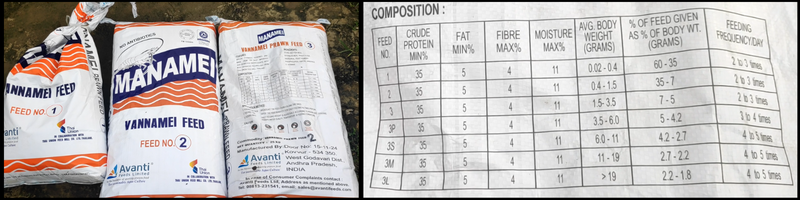
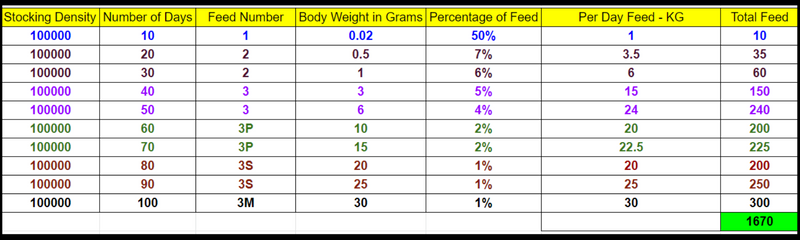
What is Alkalinity?
Aquaculture is the farming of aquatic organisms, such as fish, crustaceans, and mollusks, in controlled environments. The quality of the water in these environments is essential to the health and well-being of the aquatic organisms. One important aspect of water quality is alkalinity, which is a measure of the water’s ability to resist changes in pH.
What is Alkalinity in Aquaculture?
In aquaculture, alkalinity refers to the measurement of the water’s buffering capacity, which is its ability to neutralize acid. Alkalinity is often measured in terms of carbonate hardness, which represents the amount of calcium carbonate (CaCO3) in the water. Other compounds, such as bicarbonate (HCO3-) and hydroxide (OH-), can also contribute to alkalinity.
Alkalinity is an important factor in the health and well-being of aquatic organisms because it helps to stabilize the pH of the water. A stable pH is important because it affects many biological and chemical processes in the water, including the solubility of minerals and the availability of dissolved oxygen. Changes in pH can be stressful or even lethal to aquatic organisms, so it’s important to maintain a stable pH by managing alkalinity.
Why is Alkalinity important in Aquaculture?
Alkalinity is important in aquaculture for several reasons. First, as mentioned earlier, alkalinity helps to stabilize the pH of the water. This is important because many aquatic organisms have a narrow range of pH values in which they can survive and thrive. Changes in pH can cause stress or even death to these organisms, so maintaining a stable pH is essential.
Second, alkalinity is important because it affects the availability of minerals in the water. Many minerals, such as calcium and magnesium, are essential for the growth and development of aquatic organisms. However, these minerals can become insoluble in water when the pH is too low. Alkalinity helps to keep the pH from dropping too low, which in turn helps to keep these minerals soluble and available for uptake by the aquatic organisms.
Third, alkalinity plays a role in the breakdown of organic matter in the water. As organic matter decomposes, it produces organic acids that can lower the pH of the water. Alkalinity helps to neutralize these acids and prevent the pH from dropping too low. This, in turn, helps to maintain a healthy environment for the aquatic organisms.
Factors that Affect Alkalinity in Aquaculture
Several factors can affect alkalinity in aquaculture. One of the most important factors is the buffering capacity of the water. Water with a high buffering capacity will have a higher alkalinity and be better able to resist changes in pH. This is because water with a high buffering capacity has more carbonate, bicarbonate, and other alkaline compounds that can neutralize acid.
Another factor that can affect alkalinity is the concentration of dissolved carbon dioxide in the water. Carbon dioxide can contribute to the acidity of the water and reduce alkalinity. Changes in temperature, salinity, and other physical and chemical parameters can also affect alkalinity.
Monitoring and Managing Alkalinity in Aquaculture
To maintain optimal water quality in an aquaculture system, it is important to monitor and manage alkalinity. Regular monitoring of alkalinity can help identify potential problems early and allow for corrective action to be taken before serious damage is done to the aquatic organisms.
There are several ways to manage alkalinity in an aquaculture system. One common approach is to add buffering agents to the water, such as sodium bicarbonate or calcium carbonate. These agents can help maintain a stable alkalinity by neutralizing any excess acidity in the water.
Another approach is to manage the amount of organic matter in the system. Excessive organic matter can lead to the production of organic acids, which can lower alkalinity and destabilize the pH of the water. Managing the amount of organic matter in the system through proper feeding practices, adequate filtration, and regular water exchanges can help maintain a stable alkalinity.
Water exchanges are another important tool for managing alkalinity in an aquaculture system. When water is exchanged, it brings in fresh alkaline water and removes any excess acidity from the system. Regular water exchanges can help maintain a stable alkalinity and pH in the system.
Finally, it is important to maintain good management practices to prevent the buildup of excess acidity in the system. This can include regular cleaning of filters and other equipment, careful monitoring of feeding practices, and avoiding overstocking of the system.
Conclusion
Alkalinity is an important factor in the health and well-being of aquatic organisms in aquaculture. It helps to stabilize the pH of the water, maintain the availability of minerals, and prevent the buildup of excess acidity in the system. Monitoring and managing alkalinity in an aquaculture system is crucial to maintaining optimal water quality and ensuring the growth and survival of the aquatic organisms.
By maintaining a stable alkalinity, aquaculture professionals can create a healthy and stable environment for their aquatic organisms. This can lead to improved growth rates, better disease resistance, and overall improved performance of the system. With proper monitoring and management, alkalinity can be maintained at optimal levels, helping to ensure the long-term success of the aquaculture operation.